TOTAL QUALITY COMPANY
DMG ITALIA, a total quality company for Medical Devices, supports you with the most innovative and tailor made solutions with a strong know-how in R&D, Regulatory and Production.

read more >>
REGULATORY
 Experience in developing, registering, manufacturing and marketing CE marks in class 1, class 2A and 2B, class 3. Strongly focused on safety and quality. DMG Regulatory Department is able to grant a full support for a «ready to market» approach.
Experience in developing, registering, manufacturing and marketing CE marks in class 1, class 2A and 2B, class 3. Strongly focused on safety and quality. DMG Regulatory Department is able to grant a full support for a «ready to market» approach.

read more>>
RESEARCH & DEVELOPMENT
 Strong pipeline of innovative solutions in paediatrics, gastroenterology, ENT, ophthalmology, allergology, geriatrics and other specific therapeutic areas. Broad expertise in development of new products combining creativity with a pragmatic approach.
Strong pipeline of innovative solutions in paediatrics, gastroenterology, ENT, ophthalmology, allergology, geriatrics and other specific therapeutic areas. Broad expertise in development of new products combining creativity with a pragmatic approach.

read more>>
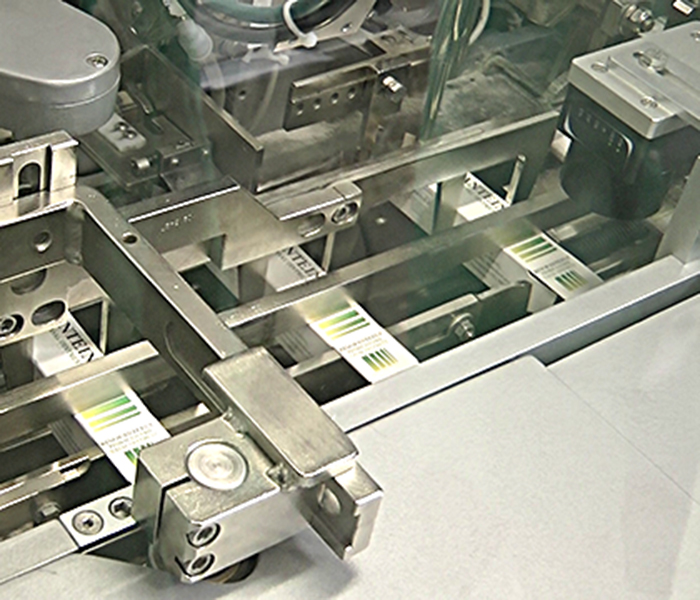
PRODUCTION
 Excellence in testing and quality control, manufacturing and packaging. DMG production site includes liquids (syrups, drops, shampoos and lotions), semisolids (creams, ointments and gels) and can manufacture Medical Devices, Nutritional Supplements and advanced cosmetics.
Excellence in testing and quality control, manufacturing and packaging. DMG production site includes liquids (syrups, drops, shampoos and lotions), semisolids (creams, ointments and gels) and can manufacture Medical Devices, Nutritional Supplements and advanced cosmetics.
After having obtained in 2002 the Approval for Quality Assurance System for production and/or sterilization, according to Annex V of EC Directive 93/42/EEC, by the “Istituto Superiore di Sanità”, Notified Body N° 0373, we have endowed our company with the ISO certifications for research and development, production and trade of Medical Devices.
ISO 9001:2008 – 04.07.2014
Design, production and control of sterile and non sterile medical devices in solution, gel, emulsion, paste, suspension, ointment, for protection and treatment for ophthalmology, otorhinolaryngology, gastroenterology, pediatrics and orthopaedics. Design and production of nutritional supplements. Trade and scientific information of sterile and non sterile medical devices, nutritional supplements and medicines.
ISO 13485:2012
Design, production and control of sterile and non sterile medical devices in solution, gel, emulsion, paste, ointment, suspension, for protection and treatment for ophthalmology, otorhinolaryngology, gastroenterology, pediatrics and orthopaedics.
OUR COMMITMENT
The well being of patients is our priority.
DMG ITALIA provides healthcare products that address the evolving needs of doctors and patients. We strongly believe that innovative solutions can improve the quality of life and patient well being is our primary goal. Focused solely on patient satisfaction, DMG ITALIA offers a diversified portfolio of the highest quality products to best improve people’s health and well being.
For us, here at DMG Italia, promoting human capital is central. Our commitment towards our employees is accomplished by providing equal employment opportunities, encouraging teamwork, listening to their ideas and delivering a comfortable and friendly working environment. We also develop training programs to enhance specific skills and offer challenging and satisfying career prospects, paving the way for personal development. In a permanently evolving company like DMG ITALIA, emphasis on trainings, to increase value added to products and services offered, is critical to respect the Company’s vision, aspirations and values and has allowed our steady growth through mutual respect and diversity.
DMG ITALIA is committed to protect the environment, continually becoming more aware of and sensitive to the environmental impact of our activities. DMG ITALIA aims to conduct business with the highest applicable legal and ethical rules and values to contribute to environmental protection. DMG ITALIA steers all its employees to better understand how they may contribute to reducing our environmental impact. We fulfill our commitment by:
- Complying with environmental regulations.
- Conducting operations in an environmentally sound manner.
- Promoting environmental responsibility among our employees.
- Purchasing environmentally friendly materials when appropriate.
- Applying the principles of reduce, re-use and recycle in all processes.
- Sharing information electronically within the office.
- Striving to ensure that suppliers agree to comply with environmental regulations.
- Pursuing continuous improvement in our environmental performance.
- Clearly communicating DMG ITALIA’s environmental policy, practices,
and impact to interested parties.
We are conscious that this list is not yet able to lay claim to completeness as regards inclusion of all details. Our Environmental Statement, however, does represent a starting platform for achieving continuous improvement of environmental performance. With this aim, we regularly review our policies, practices and processes to ensure that we continue to operate in the most sustainable way.